Have you ever stopped to think about how tiny atoms arrange themselves in space? It's a pretty fascinating idea, really. When we consider something like aluminum chloride, often written as AlCl3, its shape, or what we call its electron geometry, plays a very important part in how it behaves. This arrangement of electrons around the central aluminum atom is, you know, what gives this compound its particular personality, so to speak.
The way electrons gather around a central atom isn't just some random thing; it’s a very orderly process driven by how these negatively charged particles push away from each other. Think of it like trying to fit a few magnets together; they naturally settle into positions where they are as far apart as they can get. For AlCl3, this principle determines its basic structure, which, in a way, dictates many of its chemical features. It's almost as if the electrons are deciding the furniture layout in a very tiny room.
So, we are going to explore the spatial setup of aluminum chloride. We will look at how its electron clouds are positioned and what that means for the overall form of the molecule. This little journey will, you know, help us see why this compound acts the way it does in various chemical situations, giving us a clearer picture of its fundamental design.
Table of Contents
- What is Electron Geometry, Anyway?
- How Many Electron Groups Around Aluminum in AlCl3 Electron Geometry?
- What Does VSEPR Tell Us About AlCl3 Electron Geometry?
- Is AlCl3 Electron Geometry the Same as its Molecular Shape?
- Why Does AlCl3 Form a Dimer, and How Does that Affect its Shape?
- What are the Angles in AlCl3 Electron Geometry (and its dimer)?
- How Does This Shape Influence AlCl3's Properties?
- Where Can We See AlCl3 Electron Geometry in Action?
What is Electron Geometry, Anyway?
When we talk about electron geometry, we're really talking about the way all the electron groups, whether they are involved in bonds or just hanging out as lone pairs, arrange themselves around a central atom. It’s like figuring out the best seating arrangement for a group of people who, in some respects, really don't like to sit too close to each other. These electron groups, you see, have a negative charge, and because of that, they naturally push each other away, trying to get as far apart as they possibly can in three-dimensional space. This basic idea helps us predict the overall shape of molecules.
The main guiding idea behind this is something called VSEPR, which stands for Valence Shell Electron Pair Repulsion. It’s a bit of a mouthful, but the basic concept is quite simple: electron pairs in the outermost shell of an atom want to keep their distance. This pushing away determines the overall pattern of electron groups. So, for any given molecule, we first count up all the electron groups around the middle atom, and then we figure out the arrangement that lets them spread out the most. This initial arrangement, in a way, sets the stage for the molecule's final look.
So, when you consider a molecule, the first thing to figure out is the central atom, then count how many "regions" of electron density are around it. These regions could be single bonds, double bonds, triple bonds, or even lone pairs of electrons that aren't making a bond. Each of these counts as one electron group. The number of these groups, honestly, dictates the fundamental electron geometry. It's a pretty straightforward system once you get the hang of it, really, kind of like a simple rulebook for atomic arrangements.
How Many Electron Groups Around Aluminum in AlCl3 Electron Geometry?
To figure out the electron geometry for AlCl3, we first need to identify the central atom. In this case, it's aluminum, or Al. Then, we need to think about how many electrons are in the outermost shell of both aluminum and chlorine, you know, the ones that get involved in making connections. Aluminum, a metal from group 13, typically has three electrons ready to form connections. Chlorine, on the other hand, is a non-metal from group 17 and generally needs one more electron to feel complete, so it usually forms one connection.
When aluminum and chlorine come together to make AlCl3, the aluminum atom forms a connection with each of the three chlorine atoms. Each connection involves one electron from aluminum and one from chlorine. So, the aluminum atom ends up having three single connections around it. It's important to notice that, in this particular setup, the aluminum atom doesn't have any lone pairs of electrons just sitting there, not involved in connections. This fact is, pretty much, a very important detail for figuring out the shape.
So, if we count the electron groups around the central aluminum atom, we find there are three groups, and all of them are involved in making connections. There are no non-bonding electron pairs. This count of three electron groups around the central atom is the key piece of information we need to determine the electron geometry of AlCl3. It's basically the starting point for applying the VSEPR ideas, which, you know, helps us see the bigger picture of its shape.
What Does VSEPR Tell Us About AlCl3 Electron Geometry?
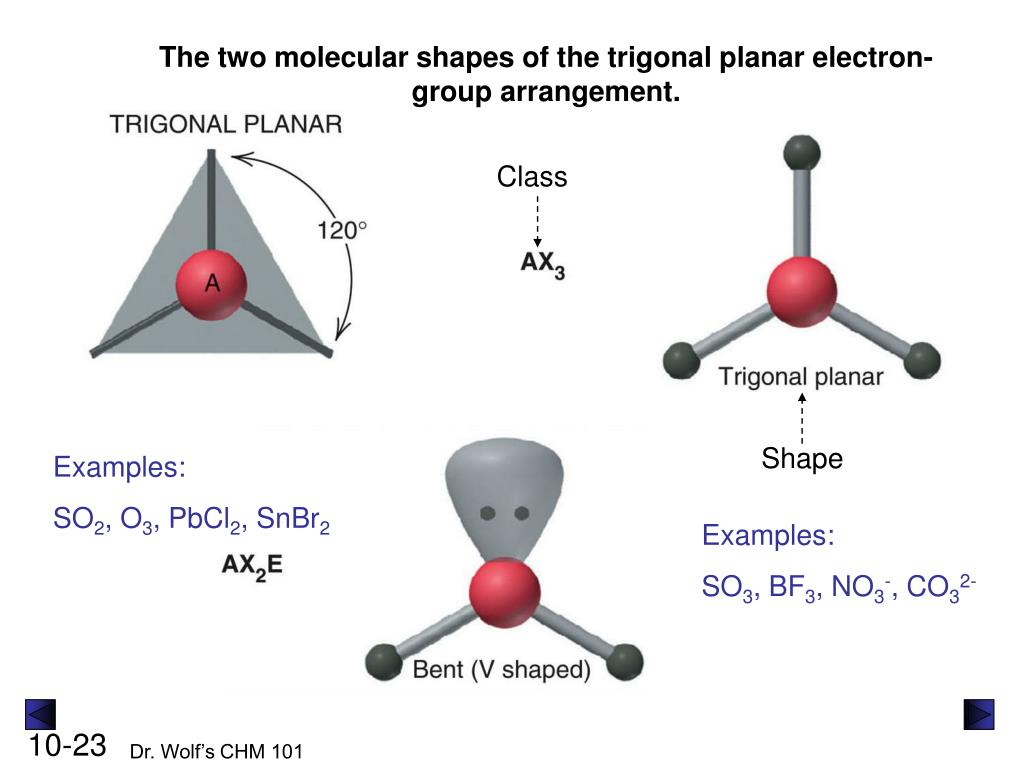
Since we've established that there are three electron groups around the central aluminum atom in AlCl3, and all of these groups are involved in making connections, the VSEPR idea tells us exactly how they will arrange themselves. With three groups, the arrangement that allows them to be as far apart as possible is a flat, three-cornered shape. This is what we call "trigonal planar" electron geometry. It's like having three spokes on a wheel, all equally spaced out around the hub, which is the aluminum atom.
In this trigonal planar setup, the ideal angle between any two connections is 120 degrees. This angle is what naturally happens when three things try to spread out evenly in a flat plane. Because there are no lone pairs of electrons on the central aluminum atom in AlCl3 to push things around differently, the electron geometry and the actual shape of the molecule are, in fact, the same. This makes it a pretty straightforward example of applying VSEPR principles, which is actually quite helpful for beginners.
So, you can picture the aluminum atom sitting right in the middle, with the three chlorine atoms connected to it, forming a perfect triangle. Each chlorine atom is, essentially, at one of the corners of this triangle. This flat, symmetrical form is a direct result of those three electron groups trying to minimize their pushing against each other. It’s a very stable and balanced arrangement, which, you know, is a common theme in how atoms prefer to connect.
Is AlCl3 Electron Geometry the Same as its Molecular Shape?
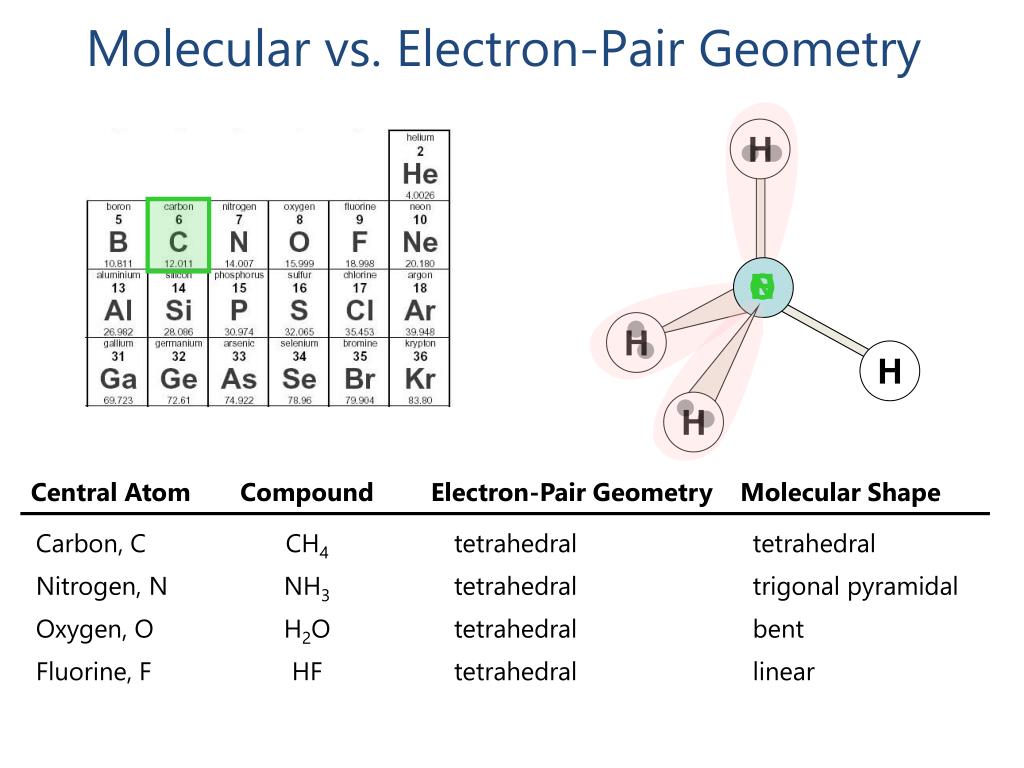
This is a really good question because electron geometry and molecular shape are sometimes different, but sometimes they are exactly the same. Electron geometry, as we discussed, looks at where all the electron groups are, including both the ones that make connections and any lone pairs. Molecular shape, on the other hand, only considers the positions of the atoms themselves, ignoring any lone pairs that might be present on the central atom. It's kind of like the difference between seeing all the furniture in a room versus just seeing where the people are sitting.
For AlCl3, the monomer form, the answer is yes, they are the same. Since the central aluminum atom has three electron groups, and all three of these groups are involved in making connections with chlorine atoms, there are no lone pairs to consider. Because there are no lone pairs, the way the electron groups arrange themselves is exactly the same as the way the atoms themselves are arranged. So, the trigonal planar electron geometry directly translates to a trigonal planar molecular shape. This makes it, you know, a pretty simple case to picture.
This situation is, basically, what happens when the central atom is surrounded only by atoms it's connected to, with no extra electron pairs floating around. The absence of these non-bonding electron pairs means there's nothing to distort the overall atomic arrangement from the ideal electron group arrangement. It's a pretty clear example of how the presence or absence of lone pairs can, you know, really change the final look of a molecule, but in this specific instance, everything lines up perfectly.
Why Does AlCl3 Form a Dimer, and How Does that Affect its Shape?
Now, here's where things get a little more interesting with AlCl3. Even though the monomer form (AlCl3) has a nice, flat, three-cornered shape, it has a bit of an issue: the central aluminum atom doesn't have a full set of eight electrons around it. It only has six electrons from its three connections. This makes it "electron deficient," and atoms, you know, really like to have that full set of eight electrons, which is called an octet. Because of this, AlCl3 is quite reactive and tends to look for more electrons.
To fix this electron deficiency, two AlCl3 molecules often join together, especially when it's in a gas form at lower temperatures or in non-polar liquids. They form a larger molecule called a dimer, which is written as Al2Cl6. In this dimer, two chlorine atoms act as bridges between the two aluminum atoms. Each bridging chlorine atom, essentially, shares its lone pairs of electrons with the electron-deficient aluminum atoms, forming what are called coordinate covalent connections. This sharing helps both aluminum atoms get closer to that desired octet, which is, you know, a very important goal for them.
When this dimer forms, the electron geometry around each aluminum atom changes. Instead of three electron groups, each aluminum atom in the Al2Cl6 dimer now has four electron groups around it – two regular connections to terminal chlorine atoms and two bridging connections to chlorine atoms shared with the other aluminum. With four electron groups, the electron geometry around each aluminum atom becomes what we call "tetrahedral." This means the connections spread out into a three-dimensional, four-sided shape, a bit like a pyramid with a triangular base. This change in shape is, basically, a pretty big deal for the molecule's overall structure and how it acts.
What are the Angles in AlCl3 Electron Geometry (and its dimer)?
Let's talk about the angles, because they really show how the atoms are positioned. In the simple AlCl3 monomer, as we've discussed, the electron geometry is trigonal planar. This means the ideal angle between any two Al-Cl connections is 120 degrees. This is the angle that allows the three chlorine atoms to be, you know, as far apart as possible while still being in a flat plane around the central aluminum atom. It's a very symmetrical and balanced arrangement, which is actually quite neat.
Now, when AlCl3 forms its dimer, Al2Cl6, the situation changes quite a bit. Each aluminum atom in the dimer is now surrounded by four electron groups, which gives it a tetrahedral electron geometry. In a perfect tetrahedron, the angles between connections are typically around 109.5 degrees. However, in the Al2Cl6 dimer, the angles are not perfectly 109.5 degrees because the two bridging chlorine atoms create some strain and distortion. The angles involving the bridging chlorine atoms are, in some respects, a little different from the angles involving the terminal chlorine atoms.
Specifically, in the Al2Cl6 dimer, you'll find two types of Cl-Al-Cl angles. The angles involving the terminal chlorine atoms are usually larger, closer to the ideal 109.5 degrees, or even a bit bigger. The angles involving the bridging chlorine atoms, the ones that connect the two aluminum atoms, are typically smaller, perhaps around 90-100 degrees. This distortion from the perfect tetrahedral angle is, you know, due to the ring structure formed by the two aluminum atoms and the two bridging chlorine atoms, and the different sizes of the atoms involved, which is actually quite interesting to consider.
How Does This Shape Influence AlCl3's Properties?
The shape of AlCl3, whether it's the monomer or the dimer, has a very direct impact on its chemical behavior. For the monomeric AlCl3, its trigonal planar shape means that the pulling of electrons towards the chlorine atoms is symmetrical. Each chlorine atom pulls electrons equally in its direction, and because they are arranged in a perfect triangle, these pulls cancel each other out. This means that the AlCl3 monomer is a nonpolar molecule. This lack of a distinct positive or negative end, you know, affects how it dissolves and interacts with other substances.
More importantly, the electron deficiency of the aluminum atom in the AlCl3 monomer, which leads to its trigonal planar shape, makes it a very strong Lewis acid. A Lewis acid is a substance that can accept a pair of electrons. Because aluminum only has six electrons around it, it's constantly looking to accept two more to complete its octet. This desire to gain electrons is a fundamental property of AlCl3 and is, basically, why it's so widely used in chemistry. Its shape, in a way, is a visual representation of its electron hunger.
When AlCl3 forms the Al2Cl6 dimer, its properties change a bit. While the dimer is still generally considered nonpolar, the slightly distorted tetrahedral arrangement around each aluminum atom means that its ability to accept electron pairs is somewhat reduced compared to the monomer. However, it still acts as a Lewis acid, just perhaps a slightly less vigorous one. The change in geometry from flat to a more three-dimensional, interconnected form, you know, really changes how it fits with other molecules and its overall reactivity. It's a pretty clear example of how form and function are tied together in chemistry.
Where Can We See AlCl3 Electron Geometry in Action?
Aluminum chloride, because of its unique electron geometry and its strong desire to accept electrons, is a pretty common player in many chemical processes, both in laboratories and in industry. Its role as a Lewis acid makes it incredibly useful for speeding up, or catalyzing, many organic chemical reactions. For instance, it's a key ingredient in what's known as the Friedel-Crafts reaction, which is a method for adding carbon groups to aromatic rings. In this reaction, the AlCl3 molecule, with its electron-hungry aluminum center, helps to create a highly reactive intermediate that drives the reaction forward. This is, you know, a pretty important industrial process.
You might also find AlCl3 used in the production of various organic compounds, including dyes and pharmaceuticals. Its ability to act as an electron acceptor means it can help break apart or rearrange other molecules, leading to the creation of new substances. The specific electron geometry of the monomer, allowing that central aluminum to be so exposed and ready to accept electrons, is what makes it so effective in these roles. So, when you see a reaction that uses AlCl3, you can, basically, picture that flat, three-cornered molecule ready to grab some electrons.
Even in more everyday applications, though perhaps less directly visible, the principles behind AlCl3's electron geometry are at play. Understanding how atoms arrange themselves in space helps scientists and engineers design new materials and processes. From creating new plastics to improving fuel efficiency, the fundamental ideas of electron geometry and molecular shape are, you know, pretty essential. So, the next time you hear about AlCl3, you can appreciate that its very specific electron arrangement is what makes it such a useful and interesting compound in the vast world of chemistry.
This arrangement truly shapes how aluminum chloride acts.Related Resources:

Detail Author:
- Name : Rod Nader DVM
- Username : ankunding.aubree
- Email : volkman.patsy@hansen.com
- Birthdate : 2002-02-03
- Address : 28428 Bertrand Divide Suite 724 Noelstad, NY 54145-0998
- Phone : +12129725250
- Company : Shanahan, Pagac and Yost
- Job : Interior Designer
- Bio : Hic inventore et sed reiciendis. Vel aspernatur tempora facilis ad non. Qui sit est unde repellat eos eos quae. A ut culpa neque perferendis et consequatur.
Socials
instagram:
- url : https://instagram.com/dare2020
- username : dare2020
- bio : Voluptates fugit sunt eos totam sunt. Doloremque numquam commodi ut facere illum alias voluptas.
- followers : 3949
- following : 2448
linkedin:
- url : https://linkedin.com/in/suzanne.dare
- username : suzanne.dare
- bio : Sit a labore praesentium aperiam qui.
- followers : 6740
- following : 2748
tiktok:
- url : https://tiktok.com/@sdare
- username : sdare
- bio : Dolorem labore doloribus animi doloremque non fugiat molestiae.
- followers : 2702
- following : 840
twitter:
- url : https://twitter.com/suzanne.dare
- username : suzanne.dare
- bio : Culpa illum quaerat possimus. Qui rerum voluptatem nisi modi ut. Quod qui aliquam autem nam est praesentium. Ea eius molestias ipsa numquam eum sed.
- followers : 4716
- following : 2534
facebook:
- url : https://facebook.com/dares
- username : dares
- bio : Optio doloribus rerum odio atque.
- followers : 6697
- following : 1873